Chapter XVI
THE MICROSCOPY OF THE COFFEE FRUIT
How the beans may be examined under the microscope, and what is revealed—Structure of the berry, the green, and the roasted bean—The coffee leaf disease under the microscope—Value of microscopic analysis in detecting adulteration
The microscopy of coffee is, on the whole, more important to the planter than to the consumer and the dealer; while, on the other hand, the microscopy is of paramount importance to the consumer and the dealer as furnishing the best means of determining whether the product offered is adulterated or not. Also, from this standpoint, the microscopy of the plant is less important than that of the bean.

Fig. 331. Coffee (Coffea arabica). I—Cross-section of berry, natural size; Pk, outer pericarp; Mk, endocarp; Ek, spermoderm; Sa, hard endosperm; Sp, soft endosperm. II—Longitudinal section of berry, natural size; Dis, bordered disk; Se, remains of sepals; Em, embryo. III—Embryo, enlarged; cot, cotyledon; rad, radicle. (Tschirch and Oesterle.)
The Fruit and the Bean
The fruit, as stated in chapter XV, consists of two parts, each one containing a single seed, or bean. These beans are flattened laterally, so as to fit together, except in the following instances: in the peaberry, where one of the ovules never develops, the single ovule, having no pressure upon it, is spherical; in the rare instances where three seeds are found, the grains are angular.
The coffee bean with which the consumer is familiar is only a small part of the fruit. The fruit, which is the size of a small cherry, has, like the cherry, an outer fleshy portion called the pericarp. Beneath this is a part like tissue paper, spoken of technically as the parchment, but known scientifically as the endocarp. Next in position to this, and covering the seed, is the so-called spermoderm, which means the seed skin, referred to in the trade as the silver skin. Small portions of this silver skin are always to be found in the cleft of the coffee bean.
The coffee bean is the embryo and its food supply; the embryo is that part of the seed which, when supplied with food and moisture, develops into a new plant. The embryo of the coffee is very minute (Fig. 331, II, Em)[101]; and the greater part of the seed is taken up by the food supply, consisting of hard and soft endosperm (Fig. 331, I and II, Sa, Sp). The minute embryo consists of two small thick leaves, the cotyledons (Fig. 331, III, cot), a short stem, invisible in the undissected embryo, and a small root, the radicle (Fig. 331, III, rad).

Fig. 332. Coffee. Cross section of beanshowing folded endosperm with hard and soft tissues. x6. (Moeller)
Fruit Structure
In order to examine the structure of these layers of the fruit under the microscope, it is necessary to use the pericarp dry, as it is not easily obtainable in its natural condition. If desired, an alcoholic specimen may be used, but it has been found that the dry method gives more satisfactory results. The dried pericarp is about 0.5 mm thick. Great difficulty is experienced in cutting microtome sections of pericarp when the specimen is embedded in paraffin, because the outer layers are soft and the endocarp is hard, and the two parts of the section separate at this point. To overcome this, the sections might also be embedded in celloidin. When the sections are satisfactory, they may be stained with any of the double stains ordinarily used in the study of plant histology.

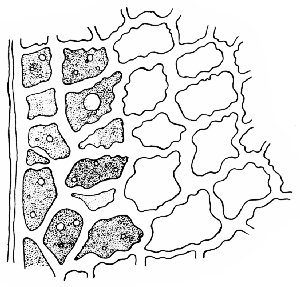
Fig. 333. Coffee. Cross section of hull and bean. Pericarp consists of: 1, epicarp; 2–3, layers of mesocarp, with 4, fibro-vascular bundle; 5, palisade layer; and 6, endocarp; ss, spermoderm, consists of 8, sclerenchyma, and 9, parenchyma; End, endosperm (Tschirch and Oesterle)
A section cut crosswise through the entire fruit would present the appearance shown in Fig. 333. The cells of the epicarp are broad and polygonal, sometimes regularly four-sided, about 15–35 µ broad. At intervals along the surface of the epicarp are stomata, or breathing pores, surrounded by guard cells. The next layer of the pericarp is the mesocarp (Figs. 333, 334, 335), the cells of which are larger and more regular in outline than the epicarp. The cells of the mesocarp become as large as 100 µ broad, but in the inner parts of the layer they become very much flattened. Fibrovascular bundles are scattered through the compressed cells of the mesocarp. The cell walls are thick; and large, amorphous, brown masses are found within the cell; occasionally, large crystals are found in the outer part of the layer. The fibro-vascular bundles consist mainly of bast and wood fibers and vessels. The bast fibers are as large as 1 mm long and 25 µ broad, with thick walls and very small lumina. Spiral and pitted vessels are also present.
The layer next to this is a soft tissue, parenchyma (Fig. 333, 5; Fig. 334, p). The parenchyma, or palisade cells as they are called, is a thin-walled tissue in which the cells are elongated, from which fact they receive their name. The walls of these cells, though very thin, are mucilaginous, and capable of taking up large amounts of water. They stain well with the aniline stains.
The endocarp (Fig. 336) is closely connected with the palisade layer and has thin-walled cells that closely resemble, in all respects, the endocarp of the apple. The outer layer consists of thick-walled fibers, which are remarkably porous (Fig. 333, 6; Fig. 336) while the fibers of the inner layer are thin-walled and run in the transverse direction.
The Bean Structure
Spermoderm, or silver skin, is not difficult to secure for microscopic analysis; because shreds of it remain in the groove of the berry, and these shreds are ample for examination. It can readily be removed without tearing, if soaked in water for a few hours. The spermoderm is thin enough not to need sectioning. It consists of two elements—sclerenchyma and parenchyma cells. (Figs. 333, 337, st, p).

Fig. 335. Coffee. Elements of pericarp in surface view. p, parenchyma; bp, parenchyma of fibro-vascular bundle; b, bast fiber; sp, spiral vessel. x160. (Moeller)
Sclerenchyma forms an uninterrupted covering in the early stages of the seed; but as the seed develops, surrounding tissues grow more rapidly than the sclerenchyma, and the cells are pushed apart and scattered. The cells occurring in the cleft of the berry are straight, narrow, and long, becoming as long as 1 mm, and resemble bast fibers somewhat. On the surface of the berry, and sometimes in the cleft, there are found smaller, thicker cells, which are irregular in outline, club-shaped and vermiform types predominating.
Parenchyma cells form the remainder of the spermoderm; and these are partially obliterated, so that the structure is not easily seen, appearing almost like a solid membrane. The raphe runs through the parenchyma found in the cleft of the berry.
The endosperm (Figs. 333; 338) consist of small cells in the outer part, and large cells, frequently as thick as 100 µ, in the inner part. The cell walls are thickened and knotted. Certain of the inner cells have mucilaginous walls which when treated with water disappear, leaving only the middle lamellae, which gives the section a peculiar appearance. The cells contain no starch, the reserve food supply being stored cellulose, protein, and aleurone grains. Various investigators report the presence of sugar, tannin, iron, salts, and caffein.
The embryo (Fig. 331, III) may be obtained by soaking the bean in water for several hours, cutting through the cleft and carefully breaking apart the endosperm. If it is now soaked in diluted alkali, the embryo protrudes through the lower end of the endosperm. It is then cleared in alkali, or in chloral hydrate. The cotyledons shown have three pairs of veins, which are slightly netted. The radicle is blunt and is about 3⁄4 mm in length, while the cotyledons are 1⁄2 mm long.
The Coffee-Leaf Disease
The coffee tree has many pests and diseases; but the disease most feared by planters is that generally referred to as the coffee-leaf disease, and by this is meant the fungoid Hemileia vastatrix, which as told in chapter XV, destroyed Ceylon's once prosperous coffee industry. As it has since been found in nearly all coffee-producing countries, it has become a nightmare in the dreams of all coffee planters. The microscope shows how the spores of this dreaded fungus, carried by the winds upon a leaf of the coffee tree, proceed to germinate at the expense of the leaf; robbing it of its nourishment, and causing it to droop and to die. A mixture of powdered lime and sulphur has been found to be an effective germicide, if used in time and diligently applied.
Value of Microscopic Analysis
The value of the microscopic analysis of coffee may not be apparent at first sight; but when one realizes that in many cases the microscopic examination is the only way to detect adulteration in coffee, its importance at once becomes apparent. In many instances the chemical analysis fails to get at the root of the trouble, and then the only method to which the tester has recourse is the examination of the suspected material under the scope. The mixing of chicory with coffee has in the past been one of the commonest forms of adulteration. The microscopic examination in this connection is the most reliable. The coffee grain will have the appearance already described. Microscopically, chicory shows numerous thin-walled parenchymatous cells, lactiferous vessels, and sieve tubes with transverse plates. There are also present large vessels with huge, well-defined pits.
 Coffee Leaf Disease (Hemileia vastatrix)
Coffee Leaf Disease (Hemileia vastatrix)
1. under surface of affected leaf, x 1⁄2; 2, section through same showing mycelium, haustoria, and a spore-cluster; 3, a spore-cluster seen from below; 4, a uredospore; 5, germinating uredospore; 6, appressorial swellings at tips of germ-tubes; 7, infection through stoma of leaf; 8, teleutospores; 9, teleutospore germinating with promycelium and sporidia; 10, sporidia and their germination (2 after Zimmermann, 3 after Delacroix, 4–10 after Ward)
Roasted date stones have been used as adulterants, and these can be detected quite readily with the aid of the microscope, as they have a very characteristic microscopic appearance. The epidermal cells are almost oblong, while the parenchymatous cells are large, irregular and contain large quantities of tannin.
Adulteration and adulterants are considered more fully in chapter XVII.

Green bean, showing the size and form of the cells as well as the drops of oil contained within their cavities. Drawn with the camera lucida, and magnified 140 diameters.

A fragment of roasted coffee under the microscope. Drawn with the camera lucida, and magnified 140 diameters.
Green and Roasted Coffee Under the Microscope
 Bogota, Green
Bogota, Green
Cross Section—Magnified 200 diameters
 Bogota, Green
Bogota, Green
Tangential—Magnified 200 diameters
 Bogota, Roasted
Bogota, Roasted
Tangential—Magnified 200 diameters
GREEN AND ROASTED BOGOTA COFFEE UNDER THE MICROSCOPE
These pictures serve to demonstrate that the coffee bean is made up of minute cells that are not broken down to any extent by the roasting process. Note that the oil globules are more prominent in the green than in the roasted product
Chapter XVII
THE CHEMISTRY OF THE COFFEE BEAN
Chemistry of the preparation and treatment of the green bean—Artificial aging—Renovating damaged coffees—Extracts—"Caffetannic acid"—Caffein, caffein-free coffee—Caffeol—Fats and oils—Carbohydrates—Roasting—Scientific aspects of grinding and packaging—The coffee brew—Soluble coffee—Adulterants and substitutes—Official methods of analysis
By Charles W. Trigg
Industrial Fellow of the Mellon Institute of Industrial Research, Pittsburgh, 1916–1920
When the vast extent of the coffee business is considered, together with the intimate connection which coffee has with the daily life of the average human, the relatively small amount of accurate knowledge which we possess regarding the chemical constituents and the physiological action of coffee is productive of amazement.
True, a painstaking compilation of all the scientific and semi-scientific work done upon coffee furnishes quite a compendium of data, the value of which is not commensurate with its quantity, because of the spasmodic nature of the investigations and the non-conclusive character of the results so far obtained. The following general survey of the field argues in favor of the promulgation of well-ordered and systematic research, of the type now in progress at several places in the United States, into the chemical behavior of coffee throughout the various processes to which it is subjected in the course of its preparation for human consumption.
Green Coffee
One of the few chemical investigations of the growing tree is the examination by Graf of flowers from 20-year-old coffee trees, in which he found 0.9 percent caffein, a reducing sugar, caffetannic acid, and phytosterol. Power and Chestnut[102] found 0.82 percent caffein in air-dried coffee leaves, but only 0.087 percent of the alkaloid in the stems of the plant separated from the leaves. In the course of a study[103] instituted for the purpose of determining the best fertilizers for coffee trees, it developed that the cherries in different stages of growth show a preponderance of potash throughout, while the proportion of P2O5 attains a maximum in the fourth month and then steadily declines.
Experiments are still in progress to ascertain the precise mineral requirements of the crop as well as the most suitable stage at which to apply them. During the first five months the moisture content undergoes a steady decrease, from 87.13 percent to 65.77 percent, but during the final ripening stage in the last month there is a rise of nearly 1 percent. This may explain the premature falling and failure to ripen of the crop on certain soils, especially in years of low rainfall. Malnutrition of the trees may result also in the production of oily beans.[104]
The coffee berry comprises about 68 percent pulp, 6 percent parchment, and 26 percent clean coffee beans. The pulp is easily removed by mechanical means; but in order to separate the soft, glutinous, saccharine parchment, it is necessary to resort to fermentation, which loosens the skin so that it may be removed easily, after which the coffee is properly dried and aged. There is first a yeast fermentation producing alcohol; and then a bacterial action giving mainly inactive lactic acid, which is the main factor in loosening the parchment. For the production of the best coffee, acetic acid fermentation (which changes the color of the bean) and temperature above 60° should be avoided, as these inhibit subsequent enzymatic action.[105]
Various schemes have been proposed for utilizing the large amount of pulp so obtained in preparing coffee for market. Most of these depend upon using the pulp as fertilizer, since fresh pulp contains 2.61 percent nitrogen, 0.81 percent P2O5, 2.38 percent potassium, and 0.57 percent calcium. One procedure[106] in particular is to mix pulp with sawdust, urine, and a little lime, and then to leave this mixture covered in a pit for a year before using. In addition to these mineral matters, the pulp also contains about 0.88 percent of caffein and 18 to 37 percent sugars. Accordingly, it has been proposed[107] to extract the caffein with chloroform, and the sugars with acidulated water. The aqueous solution so obtained is then fermented to alcohol. The insoluble portion left after extraction can be used as fuel, and the resulting ash as fertilizer.
The pulp has been dried and roasted for use in place of the berry, and has been imported to England for this purpose. It is stated that the Arabs in the vicinity of Jiddah discard the kernel of the coffee berries and make an infusion of the husk.[108]
Quality of green coffee is largely dependent upon the methods used and the care taken in curing it, and upon the conditions obtaining in shipment and storage. True, the soil and climatic conditions play a determinative rôle in the creation of the characteristics of coffee, but these do not offer any greater opportunity for constructive research and remunerative improvement than does the development of methods and control in the processes employed in the preparation of green coffee for the market.
Storage prior and subsequent to shipment, and circumstances existing during transportation, are not to be disregarded as factors contributory to the final quality of the coffee. The sweating of mules carrying bags of poorly packed coffee, and the absorption of strong foreign aromas and flavors from odoriferous substances stored in too close proximity to the coffee beans, are classic examples of damage that bear iterative mention. Damage by sea water, due more to the excessive moisture than to the salt, is not so common an occurrence now as heretofore. However, a cheap and thoroughly effective means of ethically renovating coffee which has been damaged in this manner would not go begging for commercial application.
That green coffee improves with age, is a tenet generally accepted by the trade. Shipments long in transit, subjected to the effects of tropical heat under closely battened hatches in poorly ventilated holds, have developed into much-prized yellow matured coffee. Were it not for the large capital required and the attendant prohibitive carrying charges, many roasters would permit their coffees to age more thoroughly before roasting. In fact, some roasters do indulge this desire in regard to a portion of their stock. But were it feasible to treat and hold coffees long enough to develop their attributes to a maximum, still the exact conditions which would favor such development are not definitely known. What are the optimum temperature and the correct humidity to maintain, and should the green coffee be well ventilated or not while in storage? How long should coffee be stored under the most favorable conditions best to develop it? Aging for too long a period will develop flavor at the expense of body; and the general cup efficiency of some coffees will suffer if they be kept too long.
 Portion of the Investing Membrane, Showing Its Structure
Portion of the Investing Membrane, Showing Its Structure
Drawn with the camera lucida, and magnified 140 diameters
The exact reason for improvement upon aging is in no wise certain, but it is highly probable that the changes ensuing are somewhat analogous to those occurring in the aging of grain. Primarily an undefined enzymatic and mold action most likely occurs, the nature of the enzymes and molds being largely dependent upon the previous treatment of the coffee. Along with this are a loss of moisture and an oxidation, all three actions having more evident effects with the passage of time.
Artificial Aging
In consideration of the higher prices which aged products demand, attempts have naturally been made to shorten by artificial means the time necessary for their natural production. Some of these methods depend upon obtaining the most favorable conditions for acceleration of the enzyme action; others, upon the effects of micro-organisms; and still others, upon direct chemical reaction or physical alteration of the green bean.
One of the first efforts toward artificial maturing was that of Ashcroft[109], who argued from the improved nature of coffee which had experienced a delayed voyage. His method consisted of inclosing the coffee in sweat-boxes having perforated bottoms and subjecting it to the sweating action of steam, the boxes being enclosed in an oven or room maintained at the temperature of steam.
Timby[110] claimed to remove dusts, foreign odors, and impurities, while attaining in a few hours or days a ripening effect normally secured only in several seasons. In this process, the bagged coffee is placed in autoclaves and subjected to the action of air at a pressure of 2 to 3 atmospheres and a temperature of 40° to 100° F. The temperature should seldom be allowed to rise above 150° F. The pressure is then allowed to escape and a partial vacuum created in the apparatus. This alteration of pressure and vacuum is continued until the desired maturation is obtained. Desvignes[111] employs a similar procedure, although he accomplishes seasoning by treating the coffee also with oxygen or ozone.[112] First the coffee is rendered porous by storage in a hot chamber, which is then exhausted prior to admission of the oxygen. The oxygen can be ozonized in the closed vessel while in contact with the coffee. Complete aging in a few days is claimed.
Weitzmann[113] adopts a novel operation, by exposing bags of raw coffee to the action of a powerful magnetic field, obtained with two adjustable electro-magnets. The claim that a maturation naturally produced in several years is thus obtained in 1⁄2 to 2 hours is open to considerable doubt. A process that is probably attended with more commercial success is that of Gram[114] in which the coffee is treated with gaseous nitrogen dioxid.
By far the most notable progress in this field, both scientifically and commercially, has been made by Robison[115] with his "culturing" method. Here the green coffee is washed with water, and then inoculated with selected strains of micro-organisms, such as Ochraeceus or Aspergillus Wintii. Incubation is then conducted for 6 to 7 days at 90° F. and 85 percent relative humidity. Subsequent to this incubation, the coffee is stored in bins for about ten days; after which it is tumbled and scoured. With this process it is possible to improve the cupping qualities of a coffee to a surprising degree.
Renovating Damaged Coffees
Sophistication has often been resorted to in order ostensibly to improve damaged or cheap coffee. Glazing, coloring, and polishing of the green beans was openly and covertly practised until restricted by law. The steps employed did not actually improve the coffee by any means, but merely put it into condition for more ready sale. An apparently sincere endeavor to renovate damaged coffee was made by Evans[116] when he treated it with an aqueous solution of sulphuric acid having a density of 10.5° Baumé. After agitation in this solution, the beans were washed free from acid and dried. In this manner discolorations and impurities were removed and the beans given a fuller appearance.
The addition of glucose, sucrose, lactose, or dextrin to green coffees is practised by von Niessen[117] and by Winter[118], with the object of giving a mild taste and strong aroma to "hard" coffees. The addition is accomplished by impregnating, with or without the aid of vacuum, the beans with a moderately concentrated solution of the sugar, the liquid being of insufficient quantity to effect extraction. When the solution has completely disseminated through the kernels, they are removed and dried. Upon subsequent roasting, a decided amelioration of flavor is secured.
Another method developed by von Niessen[119] comprises the softening of the outer layers of the beans by steam, cold or warm water, or brine, and then surrounding them with an absorbent paste or powder, such as china clay, to which a neutralizing agent such as magnesium oxid may be added. After drying, the clay can be removed by brushing or by causing the beans to travel between oppositely reciprocated wet cloths. In the development of this process, von Niessen evidently argued that the so-called "caffetannic acid" is the "harmful" substance in coffee, and that it is concentrated in the outer layers of the coffee beans. If these be his precepts, the question of their correctness and of the efficiency of his process becomes a moot one.
A procedure which aims at cleaning and refining raw coffee, and which has been the subject of much polemical discussion, is that of Thum[120]. It entails the placing of the green beans in a perforated drum; just covering them with water, or a solution of sodium chloride or sodium carbonate, at 65° to 70° C.; and subjecting them to a vigorous brushing for from 1 to 5 minutes, according to the grade of coffee being treated. The value of this method is somewhat doubtful, as it would not seem to accomplish any more than simple washing. In fact, if anything, the process is undesirable; as some of the extractive matters present in the coffee, and particularly caffein, will be lost. Both Freund[121] and Harnack[122] hold briefs for the product produced by this method, and the latter endeavors analytically to prove its merits; but as his experimental data are questionable, his conclusions do not carry much weight.
The Acids of Coffee
The study of the acids of coffee has been productive of much controversy and many contradictory results, few of which possess any value. The acid of coffee is generally spoken of as "caffetannic acid." Quite a few attempts have been made to determine the composition and structure of this compound and to assign it a formula. Among them may be noted those of Allen,[123] who gives it the empirical formula C14H16O7; Hlasiwetz,[124] who represents it as C15H18O8; Richter, as C30H18O16; Griebel,[125] as C18H24O10, and Cazeneuve and Haddon,[126] as C21H28O14. It is variously supposed to exist in coffee as the potassium, calcium, or magnesium salt. In regard to the physical appearance of the isolated substance there is also some doubt, Thorpe[127] describing it as an amorphous powder, and Howard[128] as a brownish, syrup-like mass, having a slight acid and astringent taste.
The chemical reactions of "caffetannic acid" are generally agreed upon. A dark green coloration is given with ferric chloride; and upon boiling it with alkalies or dilute acids, caffeic acid and glucose are formed. Fusion with alkali produces protocatechuic acid.
K. Gorter[129] has made an extensive and accurate investigation into the matter, and in reporting upon the same has made some very pertinent observations. His claim is that the name "caffetannic acid" is a misnomer and should be abandoned. The so-called "caffetannic acid" is really a mixture which has among its constituents chlorogenic acid (C32H38O19), which is not a tannic acid, and coffalic acid. Tatlock and Thompson[130] have expressed the opinion that roasted coffee contains no tannin, and that the lead precipitate contains mostly coloring matter. They found only 4.5 percent of tannin (precipitable by gelatin or alkaloids) in raw coffee.
Hanausek[131] demonstrated the presence of oxalic acid in unripe beans, and citric acid has been isolated from Liberian coffee. It also has been claimed that viridic acid, C14H20O11, is present in coffee. In addition to these, the fat of coffee contains a certain percentage of free fatty acids.
It is thus apparent that even in green coffee there is no definite compound "caffetannic acid," and there is even less likelihood of its being present in roasted coffee. The conditions, high heat and oxidation, to which coffee is subjected in roasting would suffice to decompose this hypothetical acid if it were present.
In the method of analysis for caffetannic acid (No. 24) given at the end of this chapter, there are many chances of error, although this procedure is the best yet devised. Lead acetate forms three different compounds with "caffetannic acid," so that this reagent must be added with extreme care in order to precipitate the compound desired. The precipitate, upon forming, mechanically carries down with it any fats which may be present, and which are removed from it only with difficulty. The majority of the mineral salts in the solution will come down simultaneously. All of the above-mentioned organic acids form insoluble salts with lead acetate, and there will also be a tendency toward precipitation of certain of the components of caramel, the acidic polymerization products of acrolein, glycerol, etc., and of the proteins and their decomposition products.
In view of this condition of uncertainty in composition, necessity for great care in manipulation, and ever-present danger of contamination, the significance of "caffetannic acid analysis" fades. It is highly desirable that the nomenclature relevant to this analytical procedure be changed to one, such as "lead number," which will be more truly indicative of its significance.
The Alkaloids of Coffee
In addition to caffein, the main alkaloid of coffee, trigonellin—the methylbetaine of nicotinic acid—sometimes known as caffearine, has been isolated from coffee.[132] This alkaloid, having the formula C14H16O4N2, is also found in fenugreek, Trigonella fœnum-græcum, in various leguminous plants, and in the seeds of strophanthus. When pure it forms colorless needles melting at 140° C., and, as with all alkaloids, gives a weak basic reaction. It is very soluble in water, slightly soluble in alcohol, and only very slightly soluble in ether, chloroform or benzol, so that it does not contaminate the caffein in the determination of the latter. Its effects on the body have not been studied, but they are probably not very great, as Polstorff obtained only 0.23 percent from the coffee which he examined.
Caffein, thein, trimethylxanthin, or C5H(CH3)3N4O2, in addition to being in the coffee bean is also found in guarana leaves, the kola nut, maté, or Paraguay tea, and, in small quantities, in cocoa. It is also found in other parts of these plants besides those commonly used for food purposes.
A neat test for detecting the presence of caffein is that of A. Viehoever,[133] in which the caffein is sublimed directly from the plant tissue in a special apparatus. The presence of caffein in the sublimate is verified by observing its melting point, determined on a special heating stage used in connection with a microscope.
The chief commercial source of this alkaloid is waste and damaged tea, from which it is prepared by extraction with boiling water, the tannin precipitated from the solution with litharge, and the solution then concentrated to crystallize out the caffein. It is further purified by sublimation or recrystallization from water. Coffee chaff and roaster-flue dust have been proposed as sources for medicinal caffein, but the extraction of the alkaloid from the former has not proven to be a commercial success. Several manufacturers of pharmaceuticals are now extracting caffein from roaster-flue dust, probably by an adaptation of the Faunce[134] process. The recovery of caffein from roaster-flue gases may be facilitated and increased by the use of a condenser such as proposed Ewé.[135]
Pure caffein forms long, white, silky, flexible needles, which readily felt together to form light, fleecy masses. It melts at 235–7° C. and sublimes completely at 178° C., though the sublimation starts at 120°. Salts of an unstable nature are formed with caffein by most acids. The solubility of caffein as determined by Seidell[136] is given in Table I.