Automobile Battery
Introduction:
The automobile battery is used as a source of energy in the vehicle when the engine and the alternator do not run. The lead-acid battery has been proved to be the most suitable battery for automobile use. This is particularly suitable because the cost of the battery is low and taken into account.
Requirements of the vehicle battery
The battery has various numbers of requirements, which are listed as follows:
- To provide power storage and to be able to supply it quickly enough for operating the vehicle starter motor.
- To allow the use of parking lights for a reasonable time.
- To allow operation of accessories when the engine is not running.
- To act as a swamp to damp out fluctuations of system voltage.
- To allow dynamic memory and alarm systems to remain active when the vehicle is left for a period of time.
Positioning the vehicle battery
Several basic points should be considered when choosing the location for the vehicle battery:
- Weight distribution of vehicle components.
- Proximity to the starter to reduce cable length.
- Accessibility.
- Protection against contamination.
- Ambient temperature.
- Vibration protection.
These issues will vary with the type of vehicle, intended use, average operating temperature etc. Extreme temperature conditions may require either a battery heater or a cooling fan. The potential build-up of gases from the battery may also be considered.
Lead-acid batteries
The basic construction of a 12 V lead acid battery consists of
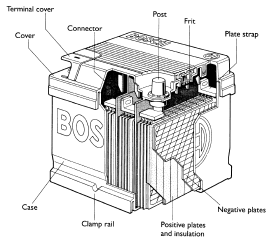
- Six cells connected in series: Each cell, producing about 2V, is housed in an individual compartment within a polypropylene; Figure shows a cut-away battery showing the main component parts.
- The active material is held in grids or baskets to form the positive and negative plates.
- Separators made from a micro porous plastic insulate these plates from each other. The grids, connecting strips and the battery posts are made from a lead alloy.
Battery rating
- The characteristics or rating of a particular battery are determined by how much current it can produce and how long it can sustain this current.
- The rate at which a battery can produce current is determined by the speed of the chemical reaction.
- This in turn is determined by a number of factors:
- Surface area of the plates.
- Temperature.
- Electrolyte strength.
- Current demanded.
Battery faults
Any electrical device can suffer from two main faults;
- These are either open circuit or short circuit.
- A battery can also suffer from other problems, such as low charge or low capacity.
- Often a problem can be traced to another part of the vehicle such as the charging system.
- Repairing modern batteries is not possible.
- Most of the problems will require the battery to be replaced.
- In the case of sulphation it is sometimes possible to bring the battery back to life with a very long low current charge. A fortieth of the ampere hour capacity or about a 1/200 of the cold start performance, for about 50 hours, is an appropriate rate.