The Ozone Layer
Introduction
The ozone layer is a crucial component of Earth's atmosphere, playing a vital role in protecting life by filtering harmful ultraviolet (UV) radiation from the Sun. Understanding the ozone layer is essential for automobile engineering students, particularly in the context of vehicle emissions and their impact on atmospheric chemistry. This detailed examination covers the formation, structure, and depletion of the ozone layer, as well as its implications for automotive engineering and environmental policy.
1. Ozone Layer Formation and Structure
1.1 Formation of Ozone
Ozone (O₃) is a molecule composed of three oxygen atoms. It is formed through a photochemical reaction involving ultraviolet (UV) radiation from the Sun:
-
Photodissociation of Oxygen:
- Reaction: UV-C radiation splits molecular oxygen (O₂) into two individual oxygen atoms.
- Result: The free oxygen atoms (O) are highly reactive and can quickly combine with other O₂ molecules.
- Reaction: UV-C radiation splits molecular oxygen (O₂) into two individual oxygen atoms.
-
Ozone Formation:
- Reaction: The free oxygen atoms react with molecular oxygen to form ozone.
- Balance: This reaction is a part of the ozone-oxygen cycle, which continuously forms and destroys ozone in the stratosphere.
- Reaction: The free oxygen atoms react with molecular oxygen to form ozone.
1.2 Ozone Layer Structure
The ozone layer is located in the stratosphere, approximately 10 to 30 kilometers above Earth's surface. Its concentration is not uniform but varies with altitude and latitude:
-
Ozone Distribution:
- Stratosphere: The highest concentrations of ozone are found in the lower stratosphere, between 15 and 35 kilometers.
- Ozone Layer Thickness: The concentration of ozone is typically measured in Dobson Units (DU), with the average thickness of the ozone layer being about 300 DU.
-
Ozone Layer Characteristics:
- Vertical Distribution: Ozone is more concentrated in the stratosphere's lower part, where it absorbs the majority of UV radiation.
- Seasonal Variations: The concentration of ozone varies seasonally, with the highest levels typically observed in the spring and fall.
2. Ozone Layer Depletion
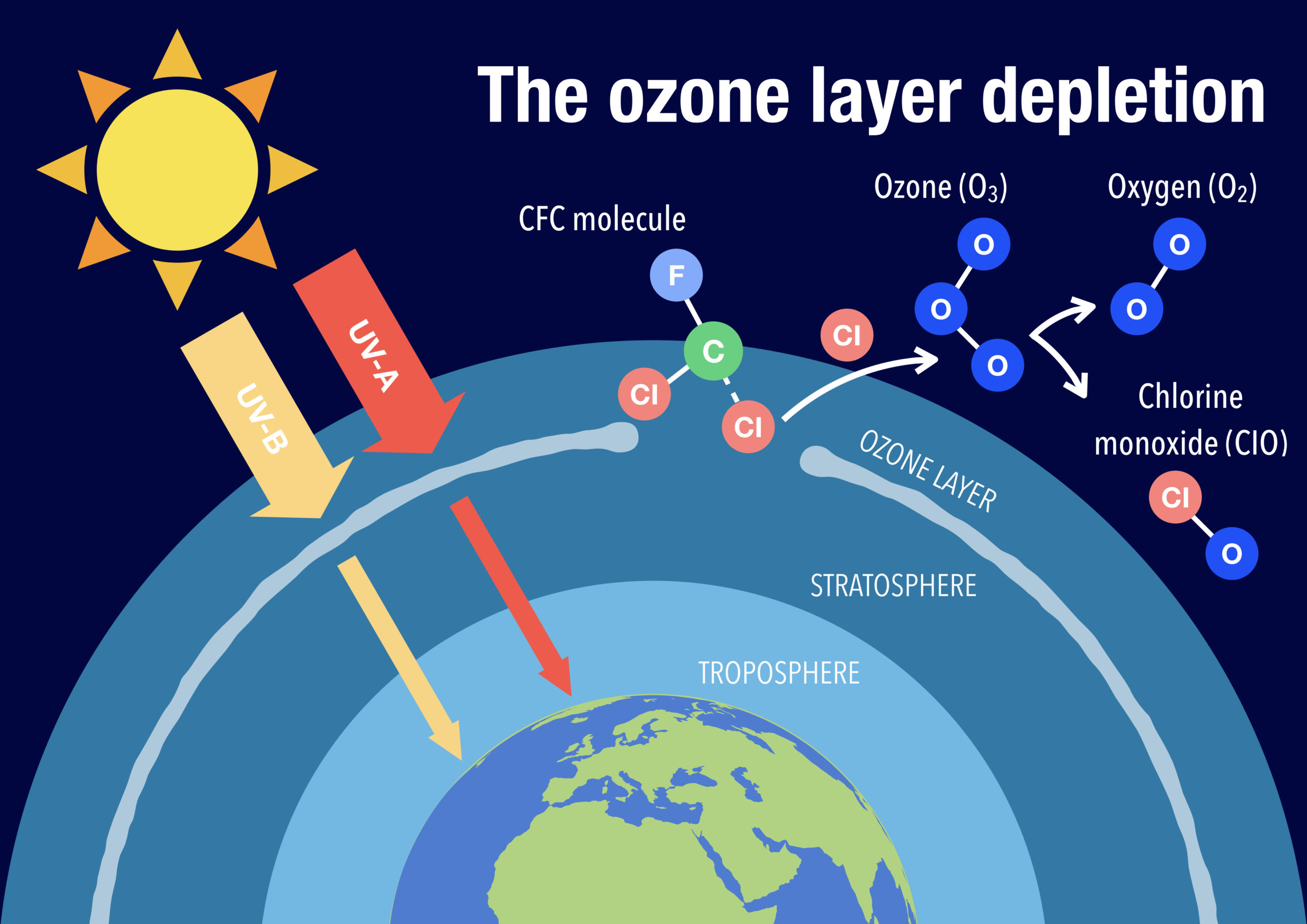
2.1 Causes of Ozone Depletion
The depletion of the ozone layer is a significant environmental concern, primarily driven by human activities and specific chemicals:
-
Chlorofluorocarbons (CFCs):
- Chemical Properties: CFCs are stable, non-reactive compounds containing chlorine and fluorine.
- Mechanism of Depletion:
- Release: CFCs are released into the atmosphere from products such as refrigerants, solvents, and aerosol propellants.
- Transport: They ascend to the stratosphere, where UV radiation breaks them down, releasing chlorine atoms.
- Ozone Destruction: Chlorine atoms react with ozone, causing its decomposition:
- Result: This catalytic process significantly reduces ozone concentrations.
-
Halons and Other Ozone-Depleting Substances:
- Halons: Used in fire extinguishers, halons release bromine atoms, which are even more efficient at destroying ozone.
- Other Substances: Methyl bromide (used in agriculture) and carbon tetrachloride also contribute to ozone depletion.
2.2 Impacts of Ozone Layer Depletion
-
Increased UV Radiation:
- Health Effects: Enhanced UV radiation leads to higher incidences of skin cancer, cataracts, and other health issues.
- Ecological Effects: UV radiation affects marine ecosystems, including phytoplankton, which forms the basis of the oceanic food chain.
-
Climate Effects:
- Temperature Changes: Depletion of the ozone layer can influence global and regional temperature patterns, potentially affecting climate systems.
-
Impact on Materials:
- Degradation: Increased UV radiation accelerates the degradation of materials such as plastics, rubber, and textiles.
3. Monitoring and Protection of the Ozone Layer
3.1 Monitoring Techniques
Monitoring the ozone layer involves a combination of ground-based and satellite observations:
-
Ground-Based Observations:
- Dobson Spectrophotometer: Measures ozone concentration by analyzing UV absorption through the atmosphere.
- Ozone Monitoring Instruments (OMI): Provide data on ozone levels using various detection methods.
-
Satellite Observations:
- Total Ozone Mapping Spectrometer (TOMS): Measures total ozone column from space, offering a global perspective on ozone distribution.
- Aura Satellite: Equipped with the Ozone Monitoring Instrument (OMI) and the Microwave Limb Sounder (MLS), it provides detailed data on ozone concentrations and related atmospheric parameters.
3.2 International Agreements and Regulations
Efforts to protect the ozone layer have led to international agreements and regulatory measures:
-
Montreal Protocol (1987):
- Objective: Aimed to phase out the production and use of ozone-depleting substances.
- Success: The protocol has been successful in reducing the emissions of CFCs, halons, and other harmful chemicals, leading to a gradual recovery of the ozone layer.
-
Kigali Amendment (2016):
- Objective: An amendment to the Montreal Protocol targeting the phase-out of hydrofluorocarbons (HFCs), which are potent greenhouse gases but do not deplete ozone directly.
- Significance: Helps mitigate climate change while addressing ozone protection.
4. Implications for Automotive Engineering
4.1 Vehicle Emissions and Ozone Depletion
Automobiles contribute to atmospheric changes through emissions that can indirectly affect ozone levels:
-
Hydrocarbons and Nitrogen Oxides (NOₓ):
- Reaction with Sunlight: Hydrocarbons and NOₓ contribute to ground-level ozone formation (smog) through photochemical reactions.
- Impact on Ozone Layer: While ground-level ozone is a pollutant, its formation is linked to precursor emissions that may also influence stratospheric ozone chemistry.
-
Regulations and Standards:
- Emission Controls: Modern regulations mandate the reduction of NOₓ and hydrocarbon emissions from vehicles, helping mitigate their impact on both ground-level ozone and stratospheric ozone.
4.2 Automotive Contributions to Climate Change
-
Greenhouse Gas Emissions:
- CO₂ and Climate Effects: Vehicle emissions contribute to greenhouse gases, which can influence global temperature and indirectly affect the ozone layer's recovery.
-
Future Technologies:
- Electric Vehicles (EVs): Adoption of EVs and alternative fuels reduces reliance on fossil fuels, potentially lowering emissions that impact both ozone and climate change.