Mechanism Of Superconductivity
Mechanism of superconductivity: Isotope effect, Tc depends on the mass of atoms
![]()
Interaction between electrons and lattice atoms is critical for the existence of superconductive state. Good conductors (weak scattering from the lattice) are poor superconductors (low TC).
Electrons on their flight through the lattice cause lattice deformation (electrons attract the positively charged lattice atoms and slightly displace them) which results in a trail of positively charged region. This positively charged region of lattice atoms attracts another electron and provides for electron-electron coupling.
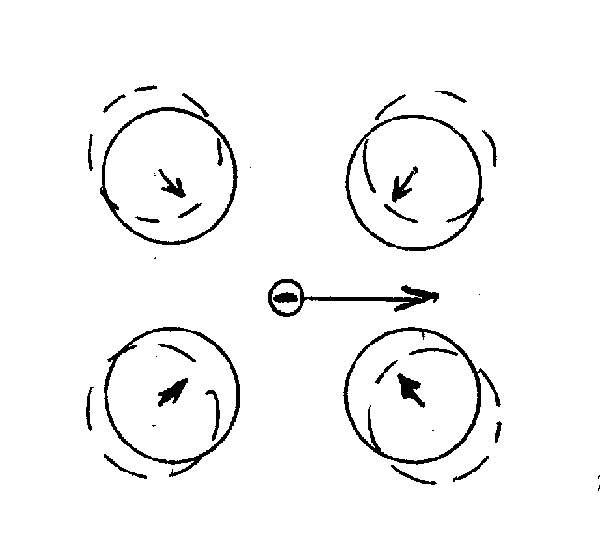
Electron pairs, and not single electrons, are charge carriers in superconductors
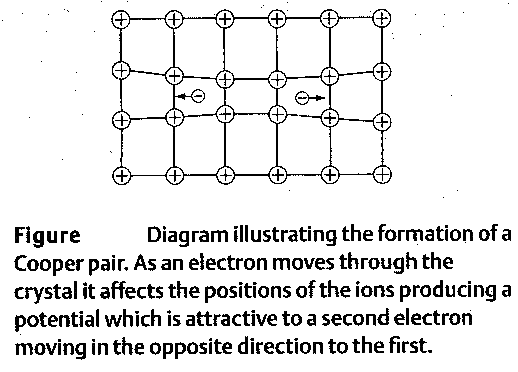
The electron-electron coupling is weak and can be destroyed by thermal motion of the lattice. For this reason superconductivity exists only at low
temperatures.
The electron-electron coupling results in electron pairing - formation of Cooper pairs. The Cooper pairs do not have spin 1/2 and therefore do not follow Pauli’s principle (1 electron per state). Large number of Cooper pairs can populate one collective state. This state is stable and requires some additional energy input (thermal energy) to be destroyed. The binding energy of Cooper pairs in the collective state is several meV.