The Importance Of Nanoscale
The Importance of Nanoscale: The Greek word "nano" (meaning dwarf) refers to a reduction of size, or time, by 10-9, which is one thousand times smaller than a micron. One nanometer (nm) is one billionth of a meter and it is also equivalent to ten Angstroms. As such a nanometer is 10-9 meter and it is 10,000 times smaller than the diameter of a human hair. A human hair diameter is about 50 micron (i.e., 50x 10-6 meter) in size, meaning that a 50
nanometer object is about 1/1000th of the thickness of a hair. One cubic nanometer (nm3) is roughly 20 times the volume of an individual atom. A
nanoelement compares to a basketball, like a basketball to the size of the earth. Figure 1 shows various size ranges for different nanoscale objects starting with such small entities like ions, atoms and molecules. Size ranges of a few nanotechnology related objects (like nanotube, single-electron transistor and quantum dot diameters) are also shown in this figure. It is obvious that nanoscience, nanoengineering and nanotechnology, all deal with very small sized objects and systems.
Officially, the United States National Science Foundation [6] defines nanoscience / nanotechnology as studies that deal with materials and systems having the following key properties.
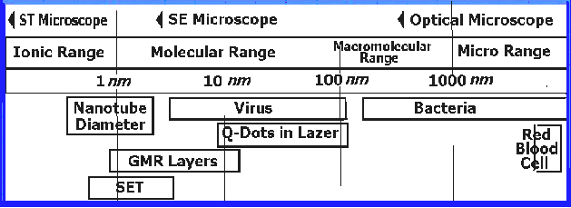
Figure 1. Comparison of size ranges for several entities as compared to some nanotechnology devices: SET (Single-electron transistor), GMR (Giant magneto resistive), Q-DOTS (Quantum dots). SE stands for Scanning Electron and ST stands for Scanning Tunneling.
- Dimension: at least one dimension from 1 to 100 nanometers (nm).
- Process: designed with methodologies that shows fundamental control over the physical and chemical attributes of molecular-scale structures.
- Building block property: they can be combined to form larger structures. Nanoscience, in a general sense, is quite natural in microbiological sciences considering that the sizes of many bioparticles dealt with (like enzymes, viruses, etc) fall within the nanometer range.
Nanoscale is a magical point on the dimensional scale: Structures in nanoscale (called nanostructures) are considered at the borderline of the smallest of human-made devices and the largest molecules of living systems. Our ability to control and manipulate nanostructures will make it possible to exploit new physical, biological and chemical properties of systems that are intermediate in size, between single atoms, molecules and bulk materials.
There are many specific reasons why nanoscale has become so important some of which are as the following
- The quantum mechanical (wavelike) properties of electrons inside matter are influenced by variations on the nanoscale. By nanoscale design of materials it is possible to vary their micro and macroscopic properties, such as charge capacity, magnetization and melting temperature, without changing their chemical composition.
- A key feature of biological entities is the systematic organization of matter on the nanoscale. Developments in nanoscience and nanotechnology would allow us to place man-made nanoscale things inside living cells. It would also make it possible to make new materials using the self-assembly features of nature. This certainly will be a powerful combination of biology with materials science.
- Nanoscale components have very high surface to volume ratio, making them ideal for use in composite materials, reacting systems, drug delivery, and chemical energy storage (such as hydrogen and natural gas).
- Macroscopic systems made up of nanostructures can have much higher density than those made up of microstructures. They can also be better conductors of electricity. This can result in new electronic device concepts, smaller and faster circuits, more sophisticated functions, and greatly reduced power consumption simultaneously by controlling nanostructure interactions and complexity.
Atomic and Molecular Basis of Nanotechnology The molecular theory of matter starts with quantum mechanics and statistical mechanics. According to the quantum mechanical Heisenberg Uncertainty Principle the position and momentum of an object cannot simultaneously and precisely be determined [8]. Then the first question that may come into mind is, how could one be able to brush aside the Heisenberg Uncertainty Principle, Figure 2, to work at the atomic and molecular level, atom by atom as is the basis of nanotechnology. The Heisenberg Uncertainty Principle helps determine the size of electron clouds, and hence the size of atoms. According to Werner Heisenberg "The more precisely the POSITION is determined, the less precisely the MOMENTUM is known". Heisenberg's Uncertainty Principle applies only to the subatomic particles like electron, positron, photon, etc. It does not forbid the possibility of nanotechnology, which has to do with the position and momentum of such large particles like atoms and molecules. This is because the mass of the atoms and molecules are quite large and the quantum mechanical calculation by the Heisenberg Uncertainty Principle places no limit on how well atoms and molecules can be held in place.
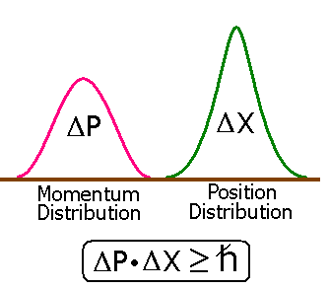
Figure 2. Heisenberg Uncertainty Principle.
Although we have long been aware of, and many investigators have been dealing with, “nano” sized entities; the historic birth of the nanotechnology is commonly credited to Feynman. Historically nanotechnology was for the first time formally recognized as a viable field of research with the landmark lecture delivered by Richard P. Feynman, the famous Noble Laureate physicist on December 29th 1959 at the annual meeting of the American Physical Society [9]. His lecture was entitled "There's Plenty of Room at the Bottom - An invitation to enter a new field of physics". Feynman stated in his lecture that the
entire encyclopedia of Britannica could be put on the tip of a needle and, in principle, there is no law preventing such an undertaking. Feynman described then the advances made in this field in the past and he envisioned the future for nanotechnology. His lecture was published in the February 1960 issue of Engineering & Science quarterly magazine of California Institute of Technology.