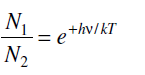Relation Between Einstein’s A And B Coefficients
RELATION BETWEEN EINSTEIN’S A AND B COEFFICIENTS: Let us consider an enclosure containing atoms which are in thermal equilibrium or in steady state. Let N1 and N2 are the number of atoms per unit volume called population in energy levels E1 and E2, respectively. Here E2 is greater than E1. In thermal equilibrium three processes of transition described above will take place.
1. Spontaneous Emission: According to Einstein the probability of spontaneous emission from energy level E2 to energy level E1 per unit time is denoted by
![]()
A21 is called the Einstein’s A coefficient of spontaneous emission of radiation. Thus the number of photons of energy E2 – E1 emitted per second by spontaneous emission in the system is equal to N2A21.
2. Induced Emission: According to Einstein the probability of induced emission from energy level E2 to energy level E1 per unit time can be written as
![]()
Here B21 is called the Einstein’s B coefficient of induced emission of radiation and u(ν) is the energy density of the radiation of frequency ν. Then the number of photons of energy hν emitted per second by induced emission in the system is equal to N2 B21 u(ν).
3. Absorption of Radiation: According to Einstein the probability of absorption of energy for transition from energy level E1 to energy level E2 per unit time can be written as
![]()
Here B12 is called the Einstein’s B coefficient of absorption of radiation and u(ν) is the energy density of the radiation of frequency ν. Then the number of photons of energy hν absorbed per second in the system is equal to N1B12 u(ν).In the thermal equilibrium state (i.e., steady state) total number of photons absorbed per second should be equal to the total number of photons emitted per second. It can be written as
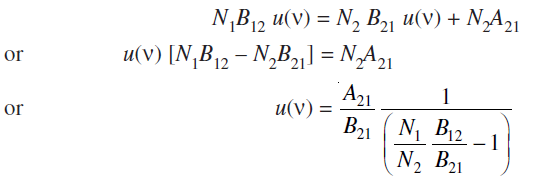 ..........eq(1)
..........eq(1)
According to Maxwell-Boltzmann distribution the number of atoms N1 and N2 in the energy states E1 and E2, respectively, in the steady state at temperature T are given by